Insulin therapy for diabetes
Scientific support: Prof. Dr. Andreas Birkenfeld, Prof. Dr. Andreas Fritsche, Prof. Dr. Michael Roden, Andreas Vosseler M.A.
In type 1 diabetes, the pancreas no longer produces enough of the blood sugar-reducing hormone insulin or stops producing it altogether. Therefore, people with the autoimmune disease type 1 diabetes are always dependent on the administration of the blood sugar-reducing hormone insulin via injection, pen, or insulin pump.
Patients with type 2 diabetes may also require insulin therapy during the course of the disease. This is the case when the body does not produce enough insulin and dietary adjustment and physical activity alone, or treatment with blood sugar-reducing tablets, are no longer effective.
Based on the current guidelines, below, we offer general information relating to the treatment of diabetes mellitus. Please note that this information does not replace consultation with a physician or contain recommendations for individual types of therapy. Please discuss individual therapy options with your doctor.
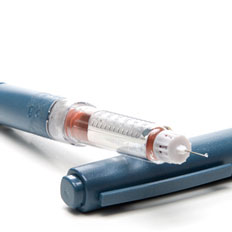
In diabetes training courses, the patients learn how to administer insulin therapy themselves on an everyday basis. The therapy itself and any adjustments should take place under the close supervision of a doctor.
The aim of the treatment is to reduce blood sugar levels and prevent the complications associated with diabetes. Permanently elevated blood sugar levels can damage the blood vessels and nerves. Medication to reduce blood sugar levels aims to prevent or delay these negative consequences as far as possible.
How does insulin work?
- In healthy people, the pancreas releases more insulin into the bloodstream when the blood sugar level rises after a meal. The insulin increase after eating is primarily triggered by the consumption of carbohydrates.
- In addition, there is a base insulin production that regulates various sugar and fat metabolism processes.
- The insulin requirement can change due to certain situations, for example, during pregnancy or when breastfeeding. Infections, accompanying diseases, or surgeries can also affect metabolism.
- The insulin requirement is normally higher in the morning and varies greatly from person to person. Many other factors, such as stress, the body’s hormones, or physical activity can influence the insulin requirement. Other nutritional components, such as fat and protein, can also influence the release of insulin.
Modern insulin preparations simulate the natural insulin release processes. They enable effective insulin therapy with few side effects.
Insulin therapy should always be accompanied by regular blood sugar self-testing. The testing helps to identify when dosage adjustment is required and counteract low or high blood sugar levels in time.
What types of insulin are available?
Nowadays, there are numerous types of insulin preparations available. Depending on their chemical structure, there is a distinction between human insulin and insulin analogs. These can then be further categorized into long-acting and short-acting insulins. There are also premixed insulins, in which a short-acting insulin is combined with a long-acting insulin.
Human insulins and insulin analogs
Insulin is a protein. Human insulins correspond to the chemical structure of natural human insulin, hence the name. They are produced by biotechnological processes using bacteria or yeasts. Insulin analogs (also known as analog insulins) are also produced using biotechnological processes. Compared to natural human insulin, the molecular structure is slightly modified, e.g., by switching or inserting individual building blocks (amino acids) at certain sections. These modifications can be used to influence the onset and duration of insulin action.
Long-acting insulins
Long-acting insulins are also known as basal insulins, intermediate insulins, or delayed insulins. They are characterized by a later onset of action and a longer duration of the effects. Long-acting human insulins are also known as human insulins with delayed release and include neutral protamine Hagedorn (NPH insulins). The onset of action of human insulins with delayed release occurs 1 to 2 hours after subcutaneous injection. The onset of the effects is delayed and lasts between 8 and 14 hours, depending on the dosage. There are also long-acting insulin analogs containing active pharmaceutical substances such as degludec, detemir, and glargin. The onset of action of long-acting insulin analogs occurs approx. 1 to 2 hours after injection. The duration of effects lasts over 19 hours and sometimes even more than 42 hours.
Short-acting insulins
Short-acting insulins are also known as bolus insulins, short-term insulins, or mealtime insulins. They are characterized by a rapid onset of action and shorter duration of their effects. Short-acting insulins are also known as regular insulins. The effects begin after about 30 minutes and last for approx. 5 to 8 hours after injection.
There are also short-acting insulin analogs with active substances such as aspart/aspart niacinamide, glulisine and lispro. Their effects begin approx. 5 to 15 minutes after injection and last for 3 to 5 hours.
What types of insulin therapy are there?
Because insulin requirements vary from person to person, insulin therapy is always tailored to the individual patient. The onset of the effects and their duration can also vary from person to person. There are various forms of insulin therapy that are now commonplace.
There are types of therapy using 2 injections per day (conventional insulin therapy) and others requiring several injections throughout the day (intensified insulin therapy). The differences between these two forms of therapy is the selected type of insulin, the injection frequency, and the insulin dosage.
When choosing the type of insulin therapy, people with diabetes should make their decision in consultation with their doctor while taking into consideration individual aspects about themselves and their current circumstances. It has been shown that, for type 1 diabetes, intensified insulin therapy is superior to conventional insulin therapy.
How does conventional insulin therapy work?
Conventional insulin therapy (CT) is mainly used in the treatment of type 2 diabetes. It can also be an option when care staff or relatives are responsible for administering the insulin therapy and the simplest possible treatment regimen is needed.
- People who use conventional insulin therapy inject a predefined amount of insulin at set times twice per day. They usually use a fixed mix of long-acting and short-acting insulins
- The effects of the insulin last the whole day. It is therefore crucial to eat meals with a certain amount of carbohydrates throughout the day. Physical activity is balanced out by eating additional meals.
- Conventional insulin therapy can be suitable for people with a very regular daily routine.
- However, the risk of suffering low blood sugar is higher when using conventional insulin therapy.
This type of therapy is not suitable if mealtimes and daily activities do not follow a fixed schedule.
What is a suitable diet during conventional insulin therapy?
People using conventional insulin therapy must adjust their food intake to the amount of insulin injected. Otherwise, they could suffer from either low or high blood sugar.
This means that, after a certain amount of time, they must consume a set amount of carbohydrates. The nutritional plan associated with conventional insulin therapy is relatively strict.
Variety is possible by changing the side dishes while ensuring identical amounts of carbohydrates are maintained. For example, they can alternate between rice, pasta, potatoes, or bread. It is important that the selected type of carbohydrate corresponds approximately to the same original amount of carbohydrates.
What does intensified insulin therapy mean?
Intensified insulin therapy (or basic-bolus therapy) is based more on the natural metabolic processes and is needs-orientated. The amount of insulin is adjusted at short notice based on current blood sugar levels, the amount of food, and physical activity. However, regular blood sugar testing is a prerequisite for this type of therapy.
- A long-acting insulin (basal insulin), injected 1-2 times per day, meets the basic insulin needs.
- And short-acting (bolus) insulin is injected before each meal to meet the increased insulin requirement.
- The insulin can be administered using an insulin pen, insulin injection, or insulin pump.
- The insulin pump is used solely with short-acting insulins. The pump administers small amounts of insulin throughout the day to meet the basic insulin needs.
Intensified insulin therapy enables better blood sugar management and is more suited to a flexible and active lifestyle. However, it requires more effort compared to conventional insulin therapy.
There are various types of intensified insulin therapy:
During intensified conventional therapy (ICT), the person administers a predefined amount of bolus insulin and basal insulin before every meal. The amount of carbohydrates in each individual meal is not additionally determined but rather follows a set plan.
In contrast, alongside the injection of basal insulin, functional insulin therapy (FIT) requires the user to calculate the amount of bolus insulin needed before each meal, based on the exact amount of carbohydrates.
During intensified conventional therapy and functional therapy, insulin is injected using an insulin pump or injection.
In contrast, during insulin pump therapy or continuous subcutaneous insulin infusion (CSII), an insulin pump is used.
How is the insulin requirement calculated?
Half of the overall insulin requirement is comprised of the basic required amount and the other half is the insulin required due to food.
The basic required amount is necessary for metabolic processes, regardless of food intake and, and is also known as the basal requirement. The aim is to achieve a constant blood sugar level while no meal is being consumed. To react to the increase in blood sugar resulting from a meal, an additional insulin dose is necessary (bolus insulin).
For type 2 diabetes, insulin therapy may be necessary when the body is not producing enough insulin and dietary adjustment and physical activity alone, or treatment using tablets, are no longer effective. For type 1 diabetes, due to the absolute insulin deficiency, insulin must always be injected. The form and amount of insulin injected vary from case to case.
For functional insulin therapy, it is essential to estimate the amount of carbohydrates and then use the calculation factor to work out the amount of insulin required for each meal. In addition, correction factors can be used to calculate the amount of needed to correct elevated blood sugar levels.
Research teams are working on developing smart insulin
The availability of short-acting and long-acting insulins has greatly simplified the treatment of diabetes. It is now possible for most users to adequately regulate their sugar metabolism. Nevertheless, people with diabetes who receive insulin therapy must test their blood sugar level before each meal, calculate the required amount of insulin and then inject the insulin.
In the future, smart or “intelligent” insulins may be able to change this. The idea is to create an insulin depot in the body to release the required amount of the hormone into the bloodstream when it is actually needed, i.e., when the blood sugar level increases. These types of insulins are also called glucose-sensitive insulins.
Smart insulins are still being researched and are not yet approved for use. Various companies and research groups have recently made progress:
- Scientists at the University of North Carolina were able to modify insulin molecules so dock onto the glucose transporters on the surface of red blood cells. As the blood sugar level rises, the insulin gradually released into the bloodstream. In diabetic mice, the researchers were able to keep sugar metabolism under control over 48 hours with just one injection of smart insulin.
- Similar results were achieved by the team at the University of Utah with an insulin derivative that uses phenylboronic acid as a glucose-sensitive “switch”.
- In another research approach, the insulin is packaged in artificial membranes that are destroyed by high sugar levels.
However, all these projects are still in a very early phase of development and testing. Experts believe that it will be several years before smart insulin comes onto the market.
Sources:
Bundesärztekammer et al.: Nationale Versorgungsleitlinie Typ-2-Diabetes. Teilpublikation der Langfassung. 2. Auflage. Version 1. 2021
Chou, D. H.-C. et al.: Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates. In: Proc Natl Acad Sci USA, 2015, 112: 2401-2406
Deutsche Diabetes Gesellschaft: S3-Leitlinie Therapie des Typ-1-Diabetes. 2. Auflage. 2018
Deutsche Diabetes Gesellschaft et al.: S3-Leitlinie Diagnostik, Therapie und Verlaufskontrolle des Diabetes mellitus im Kindes- und Jugendalter. Langfassung. 2015 (Gültigkeit abgelaufen, in Überarbeitung)
Nathan, D. M. et al.: The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 Years: Overview. In: Diabetes Care, 2014, 37: 9-16
Wang, C. et al.: Red Blood Cells for Glucose-Responsive Insulin Delivery. In: Adv Mater, 2017, 29
As of: 26.07.2021